Page 171 - Medicinal Chemistry Self Assessment
P. 171
160 Medicinal Chemistry Self Assessment
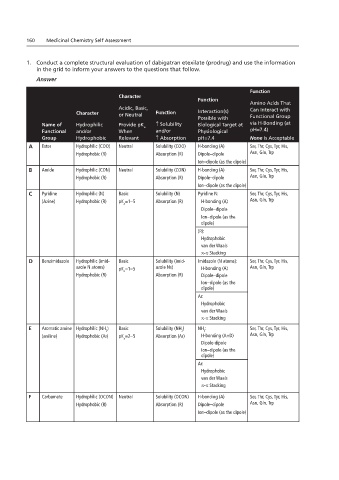
1. Conduct a complete structural evaluation of dabigatran etexilate (prodrug) and use the information
in the grid to inform your answers to the questions that follow.
Answer
Function
Character
Function
Amino Acids That
Acidic, Basic, Can Interact with
Character or Neutral Function Interaction(s)
Possible with Functional Group
Name of Hydrophilic Provide pK ↑ Solubility Biological Target at via H-Bonding (at
a
Functional and/or When and/or Physiological pH=7.4)
Group Hydrophobic Relevant ↑ Absorption pH=7.4 None Is Acceptable
A Ester Hydrophilic (COO) Neutral Solubility (COO) H-bonding (A) Ser, Thr, Cys, Tyr, His,
Hydrophobic (R) Absorption (R) Dipole–dipole Asn, Gln, Trp
Ion–dipole (as the dipole)
B Amide Hydrophilic (CON) Neutral Solubility (CON) H-bonding (A) Ser, Thr, Cys, Tyr, His,
Hydrophobic (R) Absorption (R) Dipole–dipole Asn, Gln, Trp
Ion–dipole (as the dipole)
C Pyridine Hydrophilic (N) Basic Solubility (N) Pyridine N: Ser, Thr, Cys, Tyr, His,
(Azine) Hydrophobic (R) pK =1–5 Absorption (R) H-bonding (A) Asn, Gln, Trp
a
Dipole–dipole
Ion–dipole (as the
dipole)
(R):
Hydrophobic
van der Waals
π-π Stacking
D Benzimidazole Hydrophilic (imid- Basic Solubility (imid- Imidazole (N atoms): Ser, Thr, Cys, Tyr, His,
azole N atoms) pK =1–5 azole Ns) H-bonding (A) Asn, Gln, Trp
a
Hydrophobic (R) Absorption (R) Dipole–dipole
Ion–dipole (as the
dipole)
Ar:
Hydrophobic
van der Waals
π-π Stacking
E Aromatic amine Hydrophilic (NH ) Basic Solubility (NH ) NH : Ser, Thr, Cys, Tyr, His,
2 2 2
(aniline) Hydrophobic (Ar) pK =2–5 Absorption (Ar) H-bonding (A+D) Asn, Gln, Trp
a
Dipole-dipole
Ion–dipole (as the
dipole)
Ar:
Hydrophobic
van der Waals
π-π Stacking
F Carbamate Hydrophilic (OCON) Neutral Solubility (OCON) H-bonding (A) Ser, Thr, Cys, Tyr, His,
Hydrophobic (R) Absorption (R) Dipole–dipole Asn, Gln, Trp
Ion–dipole (as the dipole)