Page 69 - Practical Technology 2025
P. 69
nd
Clinical Pharmacy-Pharm D Level Three 2 Semester 2024/2025 Pharmaceutical Technology (PT 607)
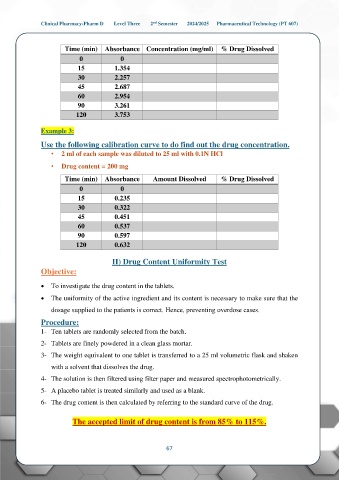
Time (min) Absorbance Concentration (mg/ml) % Drug Dissolved
0 0
15 1.354
30 2.257
45 2.687
60 2.954
90 3.261
120 3.753
Example 3:
Use the following calibration curve to do find out the drug concentration.
• 2 ml of each sample was diluted to 25 ml with 0.1N HCl
• Drug content = 200 mg
Time (min) Absorbance Amount Dissolved % Drug Dissolved
0 0
15 0.235
30 0.322
45 0.451
60 0.537
90 0.597
120 0.632
II) Drug Content Uniformity Test
Objective:
• To investigate the drug content in the tablets.
• The uniformity of the active ingredient and its content is necessary to make sure that the
dosage supplied to the patients is correct. Hence, preventing overdose cases.
Procedure:
1- Ten tablets are randomly selected from the batch.
2- Tablets are finely powdered in a clean glass mortar.
3- The weight equivalent to one tablet is transferred to a 25 ml volumetric flask and shaken
with a solvent that dissolves the drug.
4- The solution is then filtered using filter paper and measured spectrophotometrically.
5- A placebo tablet is treated similarly and used as a blank.
6- The drug content is then calculated by referring to the standard curve of the drug.
The accepted limit of drug content is from 85% to 115%.
67