Page 6 - PR 2014 2016 05 Renewable Energies
P. 6
108 Renewable Energies | Progress Report
Highlights 2014-2016
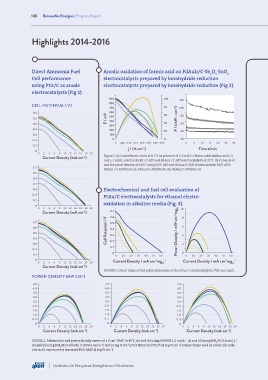
Direct Ammonia Fuel Anodic oxidation of formic acid on PdAuIr/C-Sb O ·SnO
2 5 2
Cell performance electrocatalysts prepared by borohydride reduction
using PtIr/C as anode electrocatalysts prepared by borohydride reduction (Fig 2).
electrocatalysts (Fig 1).
900 100 120
CELL POTENTIAL ( V) 800
700 80 100
0.7 600 60
500
0.6 E / mV 400 P / (mW • cm -2 ) 80
0.5 40 60
300
0.4 200 20 40
0.3 100
0. 2 0 0 20
0 0.05 0.10 0.15 0.20 0.25 0.30 0.35 0 5 10 15 20 25 30
0. 1
j / (A cm ) Time t/min
-2
0
0 2 4 6 8 10 12 14 16 18 20
Current Density (mA cm ) Figure 2. (a) Current time curves at 0. 5 V in presence of 1. 0 mol L-1 formic acid solution and 0. 5
-2
mol L-1 H SO solution for Pd / C-ATO and PdAuIr / C-ATO electrocatalysts at 25°C. (b) Curves (I-V)
2 4
and the power density at 100°C using Pd/C-ATO and PdAuIr/C-ATO electrocatalysts Pd/C-ATO;
0.7
PdAuIr / C-ATO(50:45:5); PdAuIr/C-ATO(70:20:10); PdAuIr/C-ATO(90:5:5)
0.6
0.5
0.4
0.3 Electrochemical and fuel cell evaluation of
0. 2
PtAu/C electrocatalysts for ethanol electro-
0. 1
0 oxidation in alkaline media (Fig. 3).
-1
0 2 4 6 8 10 12 14 16 18 20 10
Current Density (mA cm ) 0.7
-2
0.7 0.6 8
0.5
0.6 0.4 6
0.5 Cell Potential / V 0.3 Power Density / mW cm -2 mg Pt 4
0.4 0. 2
0.3 2
0. 1
0. 2
0 0
0. 1 0 10 20 30 40 50 60 0 10 20 30 40 50 60
0 Current Density / mA cm mg -1 Current Density / mA cm mg -1
-2
-2
0 2 4 6 8 10 12 14 16 18 20 Pt Pt
Current Density (mA cm )
-2
FIGURE 3. Direct ethanol fuel cell performance of the PtAu/C electrocatalysts, Pt/C and Au/C.
POWER DENSITY (MW CM )
-2
4.5 4.5 4.5
4.0 4.0 4.0
3.5 3.5 3.5
3.0 3.0 3.0
2.5 2.5 2.5
2. 0 2. 0 2. 0
1. 5 1. 5 1. 5
1. 0 1. 0 1. 0
0. 5 0. 5 0. 5
0 0 0
0 2 4 6 8 10 12 14 16 18 20 0 2 4 6 8 10 12 14 16 18 20 0 2 4 6 8 10 12 14 16 18 20
Current Density (mA cm ) Current Density (mA cm ) Current Density (mA cm )
-2
-2
-2
-1
-1
2
FIGURE 1. Polarization and power density curves of a 5 cm DAFC at 40°C. (a) and (b) using NH4OH 1.0 mol L . (c) and (d) using NH OH 3.0 mol L .
4
(e) and (f) using NH OH 5.0 (both in KOH 1 mol L ). Ir/C (1 mg Ir cm ), Pt/C BASF and PtIr/C (1 mg Pt cm ) compositions used as anode, for cath-
-2
-2
-1
4
-2
ode in all experiments was used Pt/C BASF (1 mg Pt cm ).
Instituto de Pesquisas Energéticas e Nucleares