Page 8 - PR 2014 2016 05 Renewable Energies
P. 8
110 Renewable Energies | Progress Report
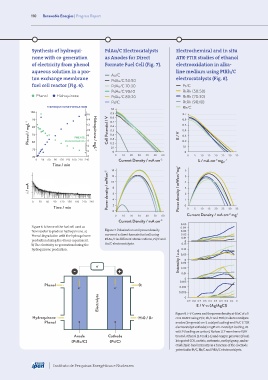
Synthesis of hydroqui- PdAu/C Electrocatalysts Electrochemical and in situ
none with co-generation as Anodes for Direct ATR-FTIR studies of ethanol
of electricity from phenol Formate Fuel Cell (Fig. 7). electrooxidation in alka-
aqueous solution in a pro- line medium using PtRh/C
Au/C
ton exchange membrane PdAu/C 50:50 electrocatalysts (Fig. 8).
fuel cell reactor (Fig. 6). PdAu/C 70:30 Pt/C
PdAu/C 90:10 RtRh (50:50)
Phenol Hidroquinone PdAu/C 80:20 RtRh (70:30)
Pd/C RtRh (90:10)
HIDROQUINONE FORMATION Rh/C
1.0
100
16 0.9 0.7
95 14 0.8 0.6
0.7
Phenol / mgL -1 90 PHENOL 10 Hidroquinone / mgL -1 Cell Potential / V 0.5 E / V 0.4
0.5
12
0.6
85
0.4
8
0.3
0.3
DEGRADATION
80
4 6 0.2 0.2
0.1
75 2 0.1 0 0
70 0 0 10 20 30 40 50 60 0 5 10 15 20 25 30 35
-2
0 30 60 90 120 150 160 210 240 Current Density / mA cm -2 L / mA cm mg pt -1
Time / min
14 6 5
Power density / mWcm -2 6 2
12
i / mA 10 8 Power density / mWcm -2 mg 1 4 3
0 30 60 90 120 150 160 210 240 4 1
Time / min 2 0 0 5 10 15 20 25 30 35
0
-2
0 10 20 30 40 50 60 Current Density / mA cm mg -1
Current Density / mA cm -2
0.05
Figure 6. Scheme of the fuel cell used as 0.04
flow reactor to produce hydroquinone. a) Figure 7. Polarization and power density 0.03
Phenol degradation with the hydroquinone curves of a direct formate fuel cell using 0.02
0.01
production during the 4 hour experiment. PdAu/C in different atomic rations, Pd/C and 0
b) The electricity co-generation during the Au/C electrocatalysts. 0.15
0.10
hydroquinone production. 0.05
Intensity / a.u. 0.03 0
e - 0.02
0.01
0
0.015
Phenol 02
e - e - 0.010
0.005 0
Electrolyte -0.9 -0.8 -0.7 -0.6 -0.5 -0.4 -0.3 -0.2 -0.1 0
E / V vs (Ag/AgCl)
Figure 8. I–V Curves and the power density at 60oC of a 5
Hydroquinone H20 / 02 cm2 DAEFC using Pt/C, Rh/C and PtRh/C electrocatalysts
Phenol anodes (1mgmetal cm-2 catalyst loading) and Pt/C E-TEK
electrocatalyst cathode(1 mgPt cm-2 catalyst loading, 20
wt% Pt loading on carbon), Nafion 117 membrane KOH
Anode Cathode treated, ethanol (2.0 mol L-1) and oxygen pressure (2 bar).
(PtRu/C) (Pt/C) Integrated CO2, acetate, carbonate, methyl group, andac-
etaldehyde band intensity as a function of the electrode
potentialfor Pt/C, Rh/C and PtRh/C electrocatalysts.
Instituto de Pesquisas Energéticas e Nucleares