Page 13 - AAS & AES & FES 01082016_Neat
P. 13
used for fluorescent measurements than for measurement of absorption. EDLs are probably the
most common source of radiation that is used for atomic fluorescence. The major disadvantage
of EDLs is the lack of availability of good commercial lamps for some elements. Commonly
available EDLs are of As, Bi, Cd, Cs, Ge, Hg, K, Pb, Rb, Sb, Se, Sn, Ti, Tl and Zn.
The intensity of the output from an EDL appears to be temperature dependent. When
using an EDL some method of temperature control is advisable, Some workers claim that the
major disadvantage to the use of an EDL is the relative instability of the lamp as compared to all
HC lamp. In many cases much of the instability is eliminated by minimal temperature control of
the lamp.
I.3.1.2.2 Hollow Cathode Lamp (HCL).
As we have already mentioned, atomic absorption lines are very narrow (about 0.002
nm). They are so narrow that if we were to use a continuous source of radiation, such as a
hydrogen or deuterium lamp, it would be very difficult to detect any absorption of the incident
radiation at all.
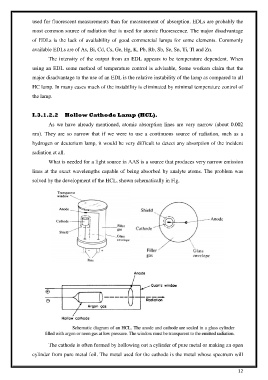
What is needed for a light source in AAS is a source that produces very narrow emission
lines at the exact wavelengths capable of being absorbed by analyte atoms. The problem was
solved by the development of the HCL, shown schematically in Fig.
The cathode is often formed by hollowing out a cylinder of pure metal or making an open
cylinder from pure metal foil. The metal used for the cathode is the metal whose spectrum will
12