Page 138 - Small Animal Internal Medicine, 6th Edition
P. 138
110 PART I Cardiovascular System Disorders
VetBooks.ir
FIG 5.7 FIG 5.8
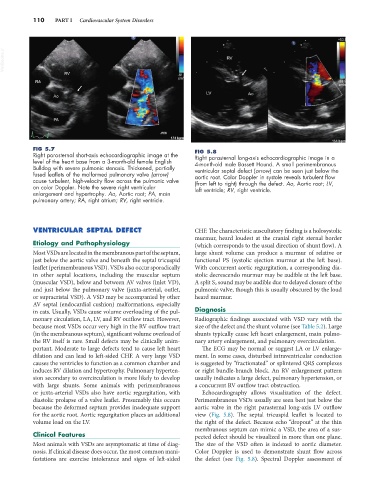
Right parasternal short-axis echocardiographic image at the Right parasternal long-axis echocardiographic image in a
level of the heart base from a 3-month-old female English 4-month-old male Bassett Hound. A small perimembranous
Bulldog with severe pulmonic stenosis. Thickened, partially ventricular septal defect (arrow) can be seen just below the
fused leaflets of the malformed pulmonary valve (arrow) aortic root. Color Doppler in systole reveals turbulent flow
cause turbulent, high-velocity flow across the pulmonic valve (from left to right) through the defect. Ao, Aortic root; LV,
on color Doppler. Note the severe right ventricular left ventricle; RV, right ventricle.
enlargement and hypertrophy. Ao, Aortic root; PA, main
pulmonary artery; RA, right atrium; RV, right ventricle.
VENTRICULAR SEPTAL DEFECT CHF. The characteristic auscultatory finding is a holosystolic
murmur, heard loudest at the cranial right sternal border
Etiology and Pathophysiology (which corresponds to the usual direction of shunt flow). A
Most VSDs are located in the membranous part of the septum, large shunt volume can produce a murmur of relative or
just below the aortic valve and beneath the septal tricuspid functional PS (systolic ejection murmur at the left base).
leaflet (perimembranous VSD). VSDs also occur sporadically With concurrent aortic regurgitation, a corresponding dia-
in other septal locations, including the muscular septum stolic decrescendo murmur may be audible at the left base.
(muscular VSD), below and between AV valves (inlet VD), A split S 2 sound may be audible due to delayed closure of the
and just below the pulmonary valve (juxta-arterial, outlet, pulmonic valve, though this is usually obscured by the loud
or supracristal VSD). A VSD may be accompanied by other heard murmur.
AV septal (endocardial cushion) malformations, especially
in cats. Usually, VSDs cause volume overloading of the pul- Diagnosis
monary circulation, LA, LV, and RV outflow tract. However, Radiographic findings associated with VSD vary with the
because most VSDs occur very high in the RV outflow tract size of the defect and the shunt volume (see Table 5.2). Large
(in the membranous septum), significant volume overload of shunts typically cause left heart enlargement, main pulmo-
the RV itself is rare. Small defects may be clinically unim- nary artery enlargement, and pulmonary overcirculation.
portant. Moderate to large defects tend to cause left heart The ECG may be normal or suggest LA or LV enlarge-
dilation and can lead to left-sided CHF. A very large VSD ment. In some cases, disturbed intraventricular conduction
causes the ventricles to function as a common chamber and is suggested by “fractionated” or splintered QRS complexes
induces RV dilation and hypertrophy. Pulmonary hyperten- or right bundle-branch block. An RV enlargement pattern
sion secondary to overcirculation is more likely to develop usually indicates a large defect, pulmonary hypertension, or
with large shunts. Some animals with perimembranous a concurrent RV outflow tract obstruction.
or juxta-arterial VSDs also have aortic regurgitation, with Echocardiography allows visualization of the defect.
diastolic prolapse of a valve leaflet. Presumably this occurs Perimembranous VSDs usually are seen best just below the
because the deformed septum provides inadequate support aortic valve in the right parasternal long-axis LV outflow
for the aortic root. Aortic regurgitation places an additional view (Fig. 5.8). The septal tricuspid leaflet is located to
volume load on the LV. the right of the defect. Because echo “dropout” at the thin
membranous septum can mimic a VSD, the area of a sus-
Clinical Features pected defect should be visualized in more than one plane.
Most animals with VSDs are asymptomatic at time of diag- The size of the VSD often is indexed to aortic diameter.
nosis. If clinical disease does occur, the most common mani- Color Doppler is used to demonstrate shunt flow across
festations are exercise intolerance and signs of left-sided the defect (see Fig. 5.8). Spectral Doppler assessment of