Page 884 - Adams and Stashak's Lameness in Horses, 7th Edition
P. 884
850 Chapter 7
VetBooks.ir
Type I
Type II
A
A
Type III
B
B
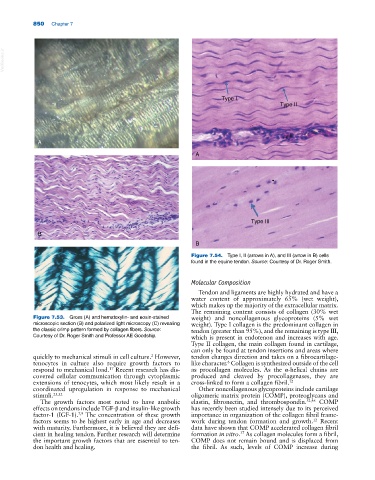
Figure 7.54. Type I, II (arrows in A), and III (arrow in B) cells
found in the equine tendon. Source: Courtesy of Dr. Roger Smith.
Molecular Composition
Tendon and ligaments are highly hydrated and have a
water content of approximately 65% (wet weight),
C which makes up the majority of the extracellular matrix.
The remaining content consists of collagen (30% wet
Figure 7.53. Gross (A) and hematoxylin‐ and eosin‐stained weight) and noncollagenous glycoproteins (5% wet
microscopic section (B) and polarized light microscopy (C) revealing weight). Type I collagen is the predominant collagen in
the classic crimp pattern formed by collagen fibers. Source: tendon (greater than 95%), and the remaining is type III,
Courtesy of Dr. Roger Smith and Professor AE Goodship. which is present in endotenon and increases with age.
Type II collagen, the main collagen found in cartilage,
can only be found at tendon insertions and areas where
quickly to mechanical stimuli in cell culture. However, tendon changes direction and takes on a fibrocartilage‐
2
6
tenocytes in culture also require growth factors to like character. Collagen is synthesized outside of the cell
respond to mechanical load. Recent research has dis as procollagen molecules. As the α‐helical chains are
17
covered cellular communication through cytoplasmic produced and cleaved by procollagenases, they are
extensions of tenocytes, which most likely result in a cross‐linked to form a collagen fibril. 12
coordinated upregulation in response to mechanical Other noncollagenous glycoproteins include cartilage
stimuli. 25,32 oligomeric matrix protein (COMP), proteoglycans and
The growth factors most noted to have anabolic elastin, fibronectin, and thrombospondin. 32,46 COMP
effects on tendons include TGF‐β and insulin‐like growth has recently been studied intensely due to its perceived
factor‐1 (IGF‐1). The concentration of these growth importance in organization of the collagen fibril frame
7,9
factors seems to be highest early in age and decreases work during tendon formation and growth. Recent
12
with maturity. Furthermore, it is believed they are defi data have shown that COMP accelerated collagen fibril
cient in healing tendon. Further research will determine formation in vitro. As collagen molecules form a fibril,
37
the important growth factors that are essential to ten COMP does not remain bound and is displaced from
don health and healing. the fibril. As such, levels of COMP increase during