Page 60 - Children Bookt.pdf
P. 60
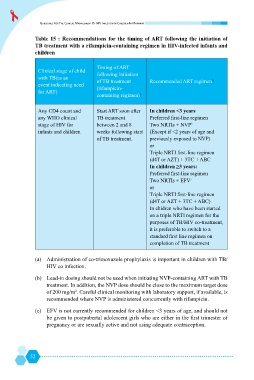
Table 15 : Recommendations for the timing of ART following the initiation of
TB treatment with a rifampicin-containing regimen in HIV-infected infants and
children
Timing of ART
Clinical stage of child
following initiation
with TB(as an
of TB treatment Recommended ART regimen b
event indicating need
(rifampicin-
for ART)
containing regimen) a
Any CD4 count and Start ART soon after In children <3 years
any WHO clinical TB treatment /
6
stage of HIV for between 2 and 8 Two NRTIs + NVP b
infants and children weeks following start @Z&
of TB treatment.
$
&
+/`
or
+!6
(d4T or AZT) + 3TC + ABC
*j"[
/
6
Two NRTIs + EFV c
or
+!6
(d4T or AZT + 3TC + ABC)
In chidren who have been started
on a triple NRTI regimen for the
purposes of TB/HIV co-treatment,
it is preferable to switch to a
completion of TB treatment
@` 6 &~
&
}"
HIV co infection.
(b) Lead-in dosing should not be used when initiating NVP-containing ART with TB
!
+/
'
&
of 200 mg/m . Careful clinical monitoring with laboratory support, if available, is
2
recommended where NVP is administered concurrently with rifampicin.
(c) EFV is not currently recommended for children <3 years of age, and should not
'
$
'
&$
;
52