Page 93 - Analytical Chemistry I E-book
P. 93
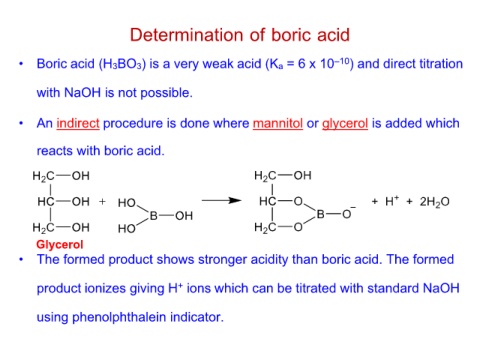
Determination of boric acid
• Boric acid (H3BO3) is a very weak acid (Ka = 6 x 10–10) and direct titration
with NaOH is not possible.
• An indirect procedure is done where mannitol or glycerol is added which
reacts with boric acid.
Glycerol
• The formed product shows stronger acidity than boric acid. The formed
product ionizes giving H+ ions which can be titrated with standard NaOH
using phenolphthalein indicator.