Page 97 - Analytical Chemistry I E-book
P. 97
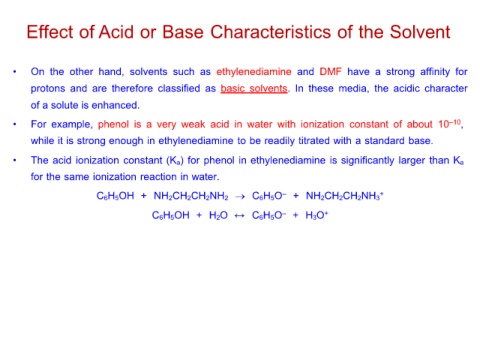
Effect of Acid or Base Characteristics of the Solvent
• On the other hand, solvents such as ethylenediamine and DMF have a strong affinity for
protons and are therefore classified as basic solvents. In these media, the acidic character
of a solute is enhanced.
• For example, phenol is a very weak acid in water with ionization constant of about 10–10,
while it is strong enough in ethylenediamine to be readily titrated with a standard base.
• The acid ionization constant (Ka) for phenol in ethylenediamine is significantly larger than Ka
for the same ionization reaction in water.
C6H5OH + NH2CH2CH2NH2 → C6H5O– + NH2CH2CH2NH3+
C6H5OH + H2O C6H5O– + H3O+