Page 99 - Analytical Chemistry I E-book
P. 99
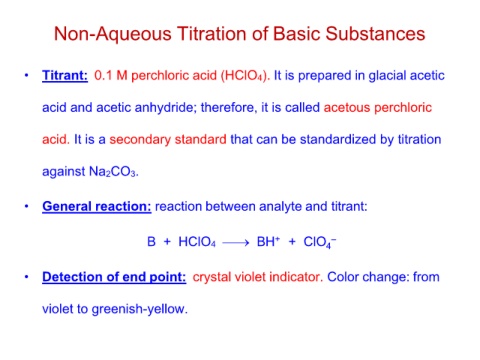
Non-Aqueous Titration of Basic Substances
• Titrant: 0.1 M perchloric acid (HClO4). It is prepared in glacial acetic
acid and acetic anhydride; therefore, it is called acetous perchloric
acid. It is a secondary standard that can be standardized by titration
against Na2CO3.
• General reaction: reaction between analyte and titrant:
B + HClO4 ⎯→ BH+ + ClO4–
• Detection of end point: crystal violet indicator. Color change: from
violet to greenish-yellow.