Page 94 - Analytical Chemistry I E-book
P. 94
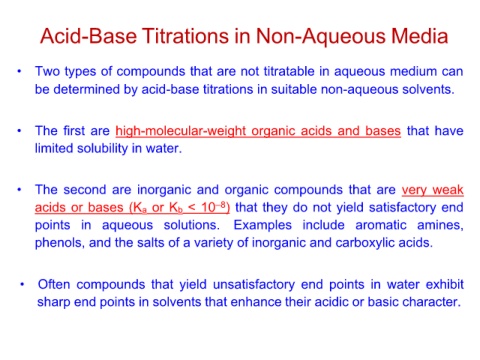
Acid-Base Titrations in Non-Aqueous Media
• Two types of compounds that are not titratable in aqueous medium can
be determined by acid-base titrations in suitable non-aqueous solvents.
• The first are high-molecular-weight organic acids and bases that have
limited solubility in water.
• The second are inorganic and organic compounds that are very weak
acids or bases (Ka or Kb < 10–8) that they do not yield satisfactory end
points in aqueous solutions. Examples include aromatic amines,
phenols, and the salts of a variety of inorganic and carboxylic acids.
• Often compounds that yield unsatisfactory end points in water exhibit
sharp end points in solvents that enhance their acidic or basic character.