Page 210 - PG 504-theoretical notes-phyto-1-2024-2025..
P. 210
Clinical pharmacy PharmD program Third level Phytochemistry-1 (PG-504)
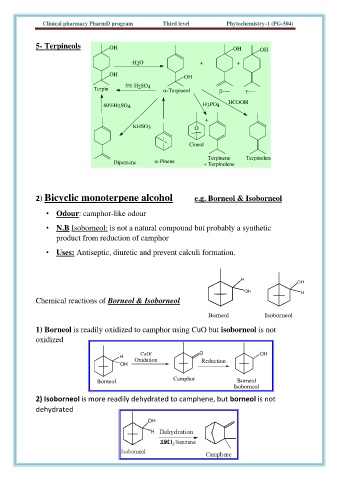
5- Terpineols OH OH OH
-H 2 O + +
OH OH
Terpin 5% H 2 SO 4 -Terpineol ---- ----
HCOOH
60%H 2 SO 4 H 3 PO 4
+
KHSO 3 O
Cineol
Terpinene Terpinolene
Dipentene -Pinene + Terpinolene
2) Bicyclic monoterpene alcohol e.g. Borneol & Isoborneol
• Odour: camphor-like odour
• N.B Isoborneol: is not a natural compound but probably a synthetic
product from reduction of camphor
• Uses: Antiseptic, diuretic and prevent calculi formation.
H
OH
OH H
Chemical reactions of Borneol & Isoborneol
Borneol Isoborneol
1) Borneol is readily oxidized to camphor using CuO but isoborneol is not
oxidized
H CuO/ O OH
Oxidation Reduction
OH
Camphor Borneol
Borneol Isoborneol
2) Isoborneol is more readily dehydrated to camphene, but borneol is not
dehydrated
191