Page 38 - E-LKM ACID BASE
P. 38
Lembar Kerja Mahasiswa - Asam Basa
-
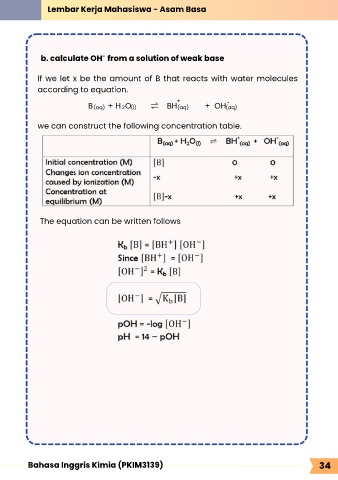
b. calculate OH from a solution of weak base
If we let x be the amount of B that reacts with water molecules
according to equation.
+ -
B + H O ⇌ BH + OH
2 (l)
(aq)
(aq)
(aq)
we can construct the following concentration table.
The equation can be written follows
Bahasa Inggris Kimia (PKIM3139) 34