Page 139 - Fluid, Electrolyte, and Acid-Base Disorders in Small Animal Practice
P. 139
Disorders of Calcium: Hypercalcemia and Hypocalcemia 129
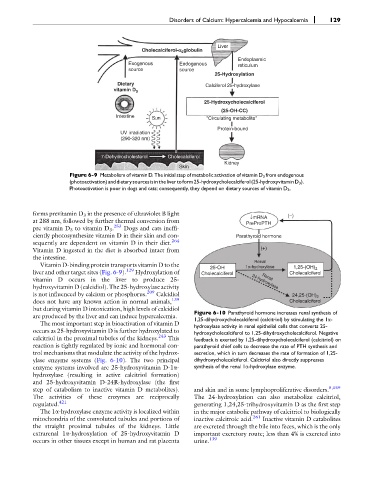
Liver
Cholecalciferol-a globulin
2
Endoplasmic
Exogenous Endogenous reticulum
source source
25-Hydroxylation
Dietary Calciferol 25-hydroxylase
vitamin D 3
25-Hydroxycholecalciferol
(25-OH-CC)
Intestine Sun "Circulating metabolite"
Protein-bound
UV irradiation
(290-320 nm)
7-Dehydrocholesterol Cholecalciferol
Kidney
Skin
Figure 6-9 Metabolism of vitamin D. The initial step of metabolic activation of vitamin D 3 from endogenous
(photoactivation)anddietarysourcesisinthelivertoform25-hydroxycholecalciferol(25-hydroxyvitaminD 3 ).
Photoactivation is poor in dogs and cats; consequently, they depend on dietary sources of vitamin D 3 .
forms previtamin D 3 in the presence of ultraviolet B light ↓mRNA (−)
at 288 nm, followed by further thermal conversion from PreProPTH
pre vitamin D 3 to vitamin D 3 . 253 Dogs and cats ineffi-
ciently photosynthesize vitamin D in their skin and con- Parathyroid hormone
sequently are dependent on vitamin D in their diet. 264
Vitamin D ingested in the diet is absorbed intact from (+)
the intestine.
Renal
Vitamin D-binding protein transports vitamin D to the 25-OH 1α-hydroxylase 1,25-(OH) 2
liver and other target sites (Fig. 6-9). 129 Hydroxylation of Cholecalciferol Cholecalciferol
vitamin D occurs in the liver to produce 25- 24-hydroxylase
Renal
hydroxyvitamin D (calcidiol). The 25-hydroxylase activity
is not influenced by calcium or phosphorus. 209 Calcidiol 24,25-(OH) 2
does not have any known action in normal animals, 139 Cholecalciferol
but during vitamin D intoxication, high levels of calcidiol Figure 6-10 Parathyroid hormone increases renal synthesis of
are produced by the liver and can induce hypercalcemia. 1,25-dihydroxycholecalciferol (calcitriol) by stimulating the 1a-
The most important step in bioactivation of vitamin D hydroxylase activity in renal epithelial cells that converts 25-
occurs as 25-hydroxyvitamin D is further hydroxylated to hydroxycholecalciferol to 1,25-dihydroxycholecalciferol. Negative
calcitriol in the proximal tubules of the kidneys. 243 This feedback is exerted by 1,25-dihydroxycholecalciferol (calcitriol) on
reaction is tightly regulated by ionic and hormonal con- parathyroid chief cells to decrease the rate of PTH synthesis and
trol mechanisms that modulate the activity of the hydrox- secretion, which in turn decreases the rate of formation of 1,25-
ylase enzyme systems (Fig. 6-10). The two principal dihydroxycholecalciferol. Calcitriol also directly suppresses
enzyme systems involved are 25-hydroxyvitamin D-1a- synthesis of the renal 1a-hydroxylase enzyme.
hydroxylase (resulting in active calcitriol formation)
and 25-hydroxyvitamin D-24R-hydroxylase (the first
step of catabolism to inactive vitamin D metabolites). and skin and in some lymphoproliferative disorders. 5,159
The activities of these enzymes are reciprocally The 24-hydroxylation can also metabolize calcitriol,
regulated. 421 generating 1,24,25-trihydroxyvitamin D as the first step
The 1a-hydroxylase enzyme activity is localized within in the major catabolic pathway of calcitriol to biologically
mitochondria of the convoluted tubules and portions of inactive calcitroic acid. 261 Inactive vitamin D catabolites
the straight proximal tubules of the kidneys. Little are excreted through the bile into feces, which is the only
extrarenal 1a-hydroxylation of 25-hydroxyvitamin D important excretory route; less than 4% is excreted into
occurs in other tissues except in human and rat placenta urine. 139