Page 135 - Fluid, Electrolyte, and Acid-Base Disorders in Small Animal Practice
P. 135
Disorders of Calcium: Hypercalcemia and Hypocalcemia 125
VDRE
silencer
cAMP-RE
enhancer
VDR Silencer
cAMP Calcitriol CaRE
controls binds CaREB
(rf1)
Start
Adapter molecules coding
RNA polymerase
region
TATA box TATA BP PTH
gene
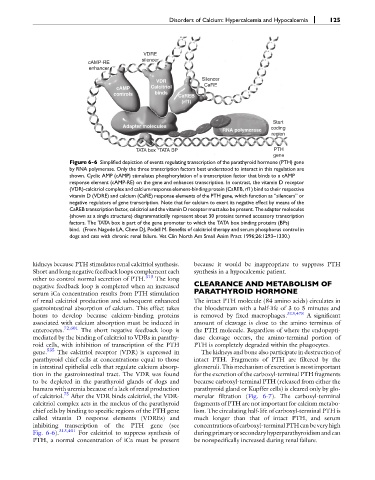
Figure 6-6 Simplified depiction of events regulating transcription of the parathyroid hormone (PTH) gene
by RNA polymerase. Only the three transcription factors best understood to interact in this regulation are
shown. Cyclic AMP (cAMP) stimulates phosphorylation of a transcription factor that binds to a cAMP
response element (cAMP-RE) on the gene and enhances transcription. In contrast, the vitamin D receptor
(VDR)-calcitriol complex and calcium response element-binding protein (CaREB, rf1) bind to their respective
vitamin D (VDRE) and calcium (CaRE) response elements of the PTH gene, which function as “silencers” or
negative regulators of gene transcription. Note that for calcium to exert its negative effect by means of the
CaREB transcription factor, calcitriol and the vitamin D receptor must also be present. The adapter molecules
(shown as a single structure) diagrammatically represent about 30 proteins termed accessory transcription
factors. The TATA box is part of the gene promoter to which the TATA box binding proteins (BPs)
bind. (From Nagode LA, Chew DJ, Podell M. Benefits of calcitriol therapy and serum phosphorus control in
dogs and cats with chronic renal failure. Vet Clin North Am Small Anim Pract 1996;26:1293–1330.)
kidneys because PTH stimulates renal calcitriol synthesis. because it would be inappropriate to suppress PTH
Short and long negative feedback loops complement each synthesis in a hypocalcemic patient.
other to control normal secretion of PTH. 313 The long
negative feedback loop is completed when an increased CLEARANCE AND METABOLISM OF
serum iCa concentration results from PTH stimulation PARATHYROID HORMONE
of renal calcitriol production and subsequent enhanced The intact PTH molecule (84 amino acids) circulates in
gastrointestinal absorption of calcium. This effect takes the bloodstream with a half-life of 3 to 5 minutes and
hours to develop because calcium-binding proteins is removed by fixed macrophages. 313,478 A significant
associated with calcium absorption must be induced in amount of cleavage is close to the amino terminus of
enterocytes. 72,601 The short negative feedback loop is the PTH molecule. Regardless of where the endopepti-
mediated by the binding of calcitriol to VDRs in parathy- dase cleavage occurs, the amino-terminal portion of
roid cells, with inhibition of transcription of the PTH PTH is completely degraded within the phagocytes.
gene. 535 The calcitriol receptor (VDR) is expressed in The kidneys and bone also participate in destruction of
parathyroid chief cells at concentrations equal to those intact PTH. Fragments of PTH are filtered by the
in intestinal epithelial cells that regulate calcium absorp- glomeruli. This mechanism of excretion is most important
tion in the gastrointestinal tract. The VDR was found for the excretion of the carboxyl-terminal PTH fragments
to be depleted in the parathyroid glands of dogs and because carboxyl-terminal PTH (released from either the
humans with uremia because of a lack of renal production parathyroid gland or Kupffer cells) is cleared only by glo-
of calcitriol. 75 After the VDR binds calcitriol, the VDR- merular filtration (Fig. 6-7). The carboxyl-terminal
calcitriol complex acts in the nucleus of the parathyroid fragments of PTH are not important for calcium metabo-
chief cells by binding to specific regions of the PTH gene lism. The circulating half-life of carboxyl-terminal PTH is
called vitamin D response elements (VDREs) and much longer than that of intact PTH, and serum
inhibiting transcription of the PTH gene (see concentrations ofcarboxyl-terminal PTH can be veryhigh
Fig. 6-6). 313,401 For calcitriol to suppress synthesis of during primaryorsecondaryhyperparathyroidism and can
PTH, a normal concentration of iCa must be present be nonspecifically increased during renal failure.