Page 171 - Withrow and MacEwen's Small Animal Clinical Oncology, 6th Edition
P. 171
150 PART II Diagnostic Procedures for the Cancer Patient
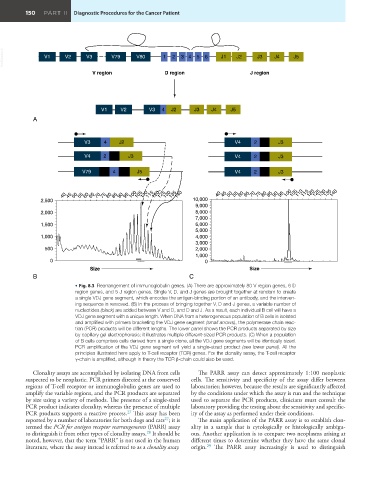
VetBooks.ir V1 V2 V3 V79 V80 1 2 34 5 6 J1 J2 J3 J4 J5
V region D region J region
V1 V2 V3 4 J2 J3 J4 J5
A
V3 4 J2 V4 2 J3
V4 2 J3 V4 2 J3
V79 4 J5 V4 2 J3
40 45 50 55 60 65 70 75 80 85 90 95 100 105 110 115 120 125 130 135 140 40 45 50 55 60 65 70 75 80 85 90 95 100 105 110 115 120 125 130 135 140
2,500 10,000
9,000
2,000 8,000
7,000
1,500 6,000
5,000
1,000 4,000
3,000
500 2,000
1,000
0 0
Size Size
B C
• Fig. 8.3 Rearrangement of immunoglobulin genes. (A) There are approximately 80 V region genes, 6 D
region genes, and 5 J region genes. Single V, D, and J genes are brought together at random to create
a single VDJ gene segment, which encodes the antigen-binding portion of an antibody, and the interven-
ing sequence is removed. (B) In the process of bringing together V, D and J genes, a variable number of
nucleotides (black) are added between V and D, and D and J. As a result, each individual B cell will have a
VDJ gene segment with a unique length. When DNA from a heterogeneous population of B cells is isolated
and amplified with primers bracketing the VDJ gene segment (small arrows), the polymerase chain reac-
tion (PCR) products will be different lengths. The lower panel shows the PCR products separated by size
by capillary gel electrophoresis; it illustrates multiple different-sized PCR products. (C) When a population
of B cells comprises cells derived from a single clone, all the VDJ gene segments will be identically sized.
PCR amplification of the VDJ gene segment will yield a single-sized product (see lower panel). All the
principles illustrated here apply to T-cell receptor (TCR) genes. For the clonality assay, the T-cell receptor
γ-chain is amplified, although in theory the TCR β-chain could also be used.
Clonality assays are accomplished by isolating DNA from cells The PARR assay can detect approximately 1:100 neoplastic
suspected to be neoplastic. PCR primers directed at the conserved cells. The sensitivity and specificity of the assay differ between
regions of T-cell receptor or immunoglobulin genes are used to laboratories; however, because the results are significantly affected
amplify the variable regions, and the PCR products are separated by the conditions under which the assay is run and the technique
by size using a variety of methods. The presence of a single-sized used to separate the PCR products, clinicians must consult the
PCR product indicates clonality, whereas the presence of multiple laboratory providing the testing about the sensitivity and specific-
27
PCR products supports a reactive process. This assay has been ity of the assay as performed under their conditions.
reported by a number of laboratories for both dogs and cats ; it is The main application of the PARR assay is to establish clon-
27
termed the PCR for antigen receptor rearrangements (PARR) assay ality in a sample that is cytologically or histologically ambigu-
to distinguish it from other types of clonality assays. It should be ous. Another application is to compare two neoplasms arising at
28
noted, however, that the term “PARR” is not used in the human different times to determine whether they have the same clonal
29
literature, where the assay instead is referred to as a clonality assay. origin. The PARR assay increasingly is used to distinguish