Page 655 - Clinical Small Animal Internal Medicine
P. 655
58 Dysbiosis and the Use of Pre‐, Pro‐ and Synbiotics 623
8
VetBooks.ir 15 6
4
10 Dysbiosis index 2
0
log CFU –2
5
–4
–6
0 –8
Blautia E. coli Faecalibact. Turicibacter Healthy IBD
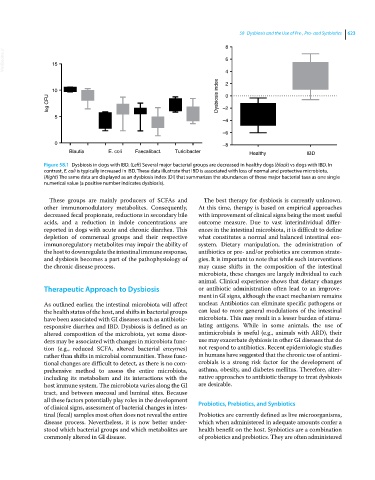
Figure 58.1 Dysbiosis in dogs with IBD. (Left) Several major bacterial groups are decreased in healthy dogs (black) vs dogs with IBD. In
contrast, E. coli is typically increased in IBD. These data illustrate that IBD is associated with loss of normal and protective microbiota.
(Right) The same data are displayed as an dysbiosis index (DI) that summarizes the abundances of these major bacterial taxa as one single
numerical value (a positive number indicates dysbiosis).
These groups are mainly producers of SCFAs and The best therapy for dysbiosis is currently unknown.
other immunomodulatory metabolites. Consequently, At this time, therapy is based on empirical approaches
decreased fecal propionate, reductions in secondary bile with improvement of clinical signs being the most useful
acids, and a reduction in indole concentrations are outcome measure. Due to vast interindividual differ-
reported in dogs with acute and chronic diarrhea. This ences in the intestinal microbiota, it is difficult to define
depletion of commensal groups and their respective what constitutes a normal and balanced intestinal eco-
immunoregulatory metabolites may impair the ability of system. Dietary manipulation, the administration of
the host to downregulate the intestinal immune response, antibiotics or pre‐ and/or probiotics are common strate-
and dysbiosis becomes a part of the pathophysiology of gies. It is important to note that while such interventions
the chronic disease process. may cause shifts in the composition of the intestinal
microbiota, these changes are largely individual to each
animal. Clinical experience shows that dietary changes
Therapeutic Approach to Dysbiosis or antibiotic administration often lead to an improve-
ment in GI signs, although the exact mechanism remains
As outlined earlier, the intestinal microbiota will affect unclear. Antibiotics can eliminate specific pathogens or
the health status of the host, and shifts in bacterial groups can lead to more general modulations of the intestinal
have been associated with GI diseases such as antibiotic‐ microbiota. This may result in a lesser burden of stimu-
responsive diarrhea and IBD. Dysbiosis is defined as an lating antigens. While in some animals, the use of
altered composition of the microbiota, yet some disor- antimicrobials is useful (e.g., animals with ARD), their
ders may be associated with changes in microbiota func- use may exacerbate dysbiosis in other GI diseases that do
tion (e.g., reduced SCFA, altered bacterial enzymes) not respond to antibiotics. Recent epidemiologic studies
rather than shifts in microbial communities. These func- in humans have suggested that the chronic use of antimi-
tional changes are difficult to detect, as there is no com- crobials is a strong risk factor for the development of
prehensive method to assess the entire microbiota, asthma, obesity, and diabetes mellitus. Therefore, alter-
including its metabolism and its interactions with the native approaches to antibiotic therapy to treat dysbiosis
host immune system. The microbiota varies along the GI are desirable.
tract, and between mucosal and luminal sites. Because
all these factors potentially play roles in the development Probiotics, Prebiotics, and Synbiotics
of clinical signs, assessment of bacterial changes in intes-
tinal (fecal) samples most often does not reveal the entire Probiotics are currently defined as live microorganisms,
disease process. Nevertheless, it is now better under- which when administered in adequate amounts confer a
stood which bacterial groups and which metabolites are health benefit on the host. Synbiotics are a combination
commonly altered in GI disease. of probiotics and prebiotics. They are often administered