Page 17 - BAHAN AJAR ANALISIS KATION
P. 17
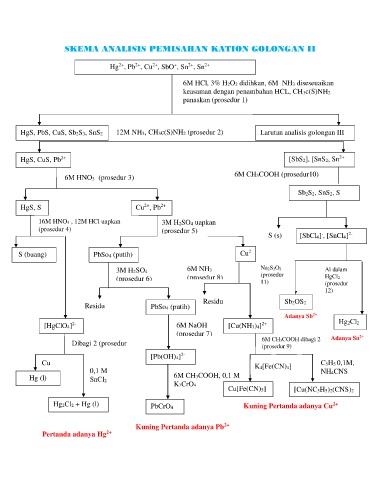
SKEMA ANALISIS PEMISAHAN KATION GOLONGAN II
2+
2+
2+
+
2+
Hg , Pb , Cu , SbO , Sn , Sn 2+
6M HCl, 3% H2O2 didihkan, 6M NH3 diseseuaikan
keasaman dengan penambahan HCL, CH3c(S)NH2
panaskan (prosedur 1)
HgS, PbS, CuS, Sb2S3, SnS2 12M NH3, CH3c(S)NH2 (prosedur 2) Larutan analisis golongan III
HgS, CuS, Pb 2+ [SbS2], [SnS2, Sn 2+
6M HNO3 (prosedur 3) 6M CH3COOH (prosedur10)
Sb2S2, SnS2, S
2+
HgS, S Cu , Pb 2+
16M HNO3 , 12M HCl uapkan 3M H2SO4 uapkan
(prosedur 4) (prosedur 5)
S (s) [SbCl4] , [SnCl4] 2-
-
S (buang) PbSo4 (putih) Cu 2
3M H2SO4 6M NH3 Na2S2O 2 Al dalam
(prosedur
(prosedur 6) (prosedur 8) 11) HgCl2
(prosedur
12)
Residu Sb2OS2
Residu PbSo4 (putih)
Adanya Sb 2+
[HgClO4] 2- 6M NaOH [Cu(NH3)4] 2+ Hg2Cl2
(prosedur 7) 6M CH 3COOH dibagi 2 Adanya Sn 2+
Dibagi 2 (prosedur (prosedur 9)
5)
[Pb(OH)4] 2-
Cu K4[Fe(CN)4] C5H5 0,1M,
0,1 M NH4CNS
Hg (l) SnCl2 6M CH3COOH, 0,1 M
K2CrO4
Cu[Fe(CN)5] [Cu(NC5H5)2(CNS)2
Hg2Cl2 + Hg (l) PbCrO4 2+ Kuning Pertanda adanya Cu 2+
Kuning Pertanda adanya Pb 2+
Pertanda adanya Hg 2+