Page 117 - Science
P. 117
RESEARCH
NEUROPHYSIOLOGY To identify candidates encoding such a proton
channel, we compared the transcriptome of mouse
2+
An evolutionarily conserved gene TRCs positive for the inward-conducting Zn -
sensitive proton current (PKD2L1 cells) with that
of TRCs that lack the current (TRPM5 cells; Fig. 1A).
family encodes proton-selective We selected genes that were enriched in PKD2L1
cells and that encoded poorly characterized or
ion channels uncharacterized transmembrane proteins (Fig. 1A
and table S1) (see methods). We expressed the can-
didates in human embryonic kidney 293 (HEK-293)
1
1
1
1
Yu-Hsiang Tu, Alexander J. Cooper, *† Bochuan Teng, * Rui B. Chang, *‡ cells or Xenopus oocytes and measured ionic cur-
Daniel J. Artiga, Heather N. Turner, Eric M. Mulhall, § Wenlei Ye, ∥ rents in response to lowering the extracellular
1
1
1
1
+
2
Andrew D. Smith, Emily R. Liman 1,3# pH (pH o ) in the absence of extracellular Na .
Of the 41 cDNAs tested, only Otopetrin1 (Otop1),
which encodes a protein (OTOP1) with 12 pre-
Ion channels form the basis for cellular electrical signaling. Despite the scores of dicted transmembrane domains (12), generated
genetically identified ion channels selective for other monatomic ions, only one type of 2+
large Zn -sensitive inward currents in response
proton-selective ion channel has been found in eukaryotic cells. By comparative
to extracellular acidification (Fig. 1B).
transcriptome analysis of mouse taste receptor cells, we identified Otopetrin1 (OTOP1), a
We characterized functional properties of OTOP1
protein required for development of gravity-sensing otoconia in the vestibular system, as
expressed in Xenopus oocytes. Unless otherwise
forming a proton-selective ion channel. We found that murine OTOP1 is enriched in
noted, the extracellular solution used in recordings
acid-detecting taste receptor cells and is required for their zinc-sensitive proton conductance.
Two related murine genes, Otop2 and Otop3,and a Drosophila ortholog also encode proton
channels. Evolutionary conservation of the gene family and its widespread tissue distribution
suggest a broad role for proton channels in physiology and pathophysiology. 1 Department of Biological Sciences, Section of
Neurobiology, University of Southern California, Los Downloaded from
2
Angeles, CA 90089, USA. Department of Biological
on channels include a large and diverse group an essential step in the replication of the virus Sciences, Section of Molecular and Computational Biology,
of membrane proteins that rapidly, and with (5). Theonlyproton-selectiveion channel iden- University of Southern California, Los Angeles, CA 90089,
3
USA. Bridge Institute, University of Southern California,
great selectivity, move ions across the cell tified in eukaryotes is the voltage-gated Hv1 (6–8), Los Angeles, CA 90089, USA.
membrane, performing crucial roles in cell which is present in immune cells, where it extrudes *These authors contributed equally to this work. †Present address:
I signaling and homeostasis (1). Ion channels protons into the phagosome to inactivate infec- Zilkha Neurogenetic Institute, Department of Cell and Neuro-
selective for each of the physiologically relevant tiousagents(9). Functional evidence indicates that biology, Keck School of Medicine, University of Southern California,
Los Angeles, CA 90089, USA. ‡Present address: Department of
+
–
2+
+
ions, Na ,K ,Ca ,and Cl , have been described ion channels that selectively transport protons Neuroscience and Department of Cellular and Molecular Physiol-
at the molecular and structural levels (2, 3), but into eukaryotic cells must also exist. For example, ogy, Yale University School of Medicine, New Haven, CT 06520, http://science.sciencemag.org/
only a few types of proton-selective ion channels in acid-sensing taste receptor cells (TRCs), an USA. §Present address: Department of Neurobiology, Harvard
2+
(proton channels) have been described (4). One inward-conducting Zn -sensitive proton current Medical School, Boston, MA 20115, USA. ||Present address:
Department of Physiology, University of California, San Francisco,
is the 96–amino acid M2 protein of influenza A, that is biophysically distinct from currents carried CA 94158, USA.
which conducts protons into the virion interior, by Hv1 has been described (10, 11). #Corresponding author. Email: liman@usc.edu
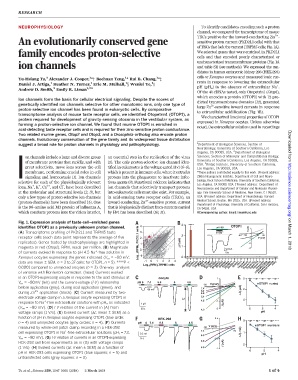
Fig. 1. Expression analysis of taste-cell–enriched genes 3
identifies OTOP1 as a previously unknown proton channel. 4 **** pH 4.5 OTOP1 I (A) µ 1
(A) Transcriptome profiling of PKD2L1 and TRPM5 taste 3 10 Zn 2+ on March 1, 2018
80
receptor cells (each data point represents the average of five (RPM), PKD2L1 cells 2 2 -80 -40 -1 40 V (mV)
replicates). Genes tested by electrophysiology are highlighted in 1 ΔI (-µA) -2
magenta or red (Otop1). RPM, reads per million. (B) Magnitude 0 1 500nA -3
+
of currents evoked in response to pH 4.5 Na -free solution in 10s
Xenopus oocytes expressing the genes indicated (V m = –80 mV; Log 10 -1 0
data are mean ± SEM, n = 3 to 37 cells; for OTOP1, n = 5). ****P< -1 0 1 2 3 4 Lrrc8c Pebp4 Slc7a5 Tmc1 uninj
Tmem163
Tmem171
Slc22a15
Slc16a12
Slc38a11
Tmem91
9130409123Rik
A630081J09Rik
Log (RPM),TRPM5 cells Abcb10 Otop1 Izumo1 Ms4a15 Slc12a8 Slc22a5 Slc35d3 Slc38a3 Slc38a5 Slc39a14 Spaca1 Tmem47 Tmem117 Tmem164 Tmem252
0.0001 compared to uninjected oocytes (n = 3). One-way analysis 10 1700028J19Rik
of variance with Bonferroni correction. (Inset) Currents evoked
in an OTOP1-expressing oocyte in response to the acid stimulus at oocyte 1 0
V m = –80mV (left) and the current-voltage (I-V) relationship pH 6 7.4 5.5 5 4.5 OTOP1 uninj
-80 V (mV)
before application (gray), during acid application (green), and
-80 mV 7.4 I (-µA) 2
during Zn 2+ application (black). (C) Current measured by two- 6.0 -1 Δ
5.5
electrode voltage clamp in a Xenopus oocyte expressing OTOP1 in I Δ 5.0 4
+
response to Na -free extracellular solutions with pH o as indicated 1 µA 4.5 -2 I (µA)
(V m = –80 mV). (D) I-V relation of the current in (A) from 30 s 4.0 -3 6 5 4
voltage ramps (1 V/s). (E) Evoked current (DI; mean ± SEM) as a pH
function of pH in Xenopus oocytes expressing OTOP1 (blue circle;
HEK-293 0.5 0
n = 4) and uninjected oocytes (gray circles; n =4). (F) Currents pH 6 7.4 5.5 5 4.5
measured by whole-cell patch clamp recording in a HEK-293 -80 V (mV) OTOP1 untrans
+
cell expressing OTOP1 in Na -free extracellular solutions (pH i =7.3, 0.5 nA 7.4 80 I (-nA) 1
V m = –80 mV). (G) I-V relation of currents in an OTOP1-expressing 5s 6.0 -0.5 Δ
HEK-293 cell from experiments as in (G) with voltage ramps 5.5 I (nA) 2
(1 V/s). (H) Evoked currents (DI; mean ± SEM) as a function of 5.0 -1
6 5 4
pH in HEK-293 cells expressing OTOP1 (blue squares; n =5)and pH
untransfected cells (gray squares; n =3).
Tu et al., Science 359, 1047–1050 (2018) 2 March 2018 1of4