Page 119 - Science
P. 119
RESEARCH | REPORT
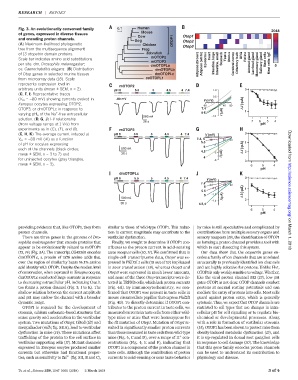
Fig. 3. An evolutionarily conserved family 0.1 Human 2048
Mouse
of genes, expressed in diverse tissues Cow Otop1
and encoding proton channels.
Dog OTOP1 Otop2
(A) Maximum-likelihood phylogenetic Chicken Otop3
tree from the multisequence alignment Frog
of 13 otopetrin domain proteins. Zebrafish
mOTOP2 Heart Testis Uterus DRG Retina
Scale bar indicates amino acid substitutions Epidermis Stomach Mast cells
mOTOP3
per site. dm, Drosophila melanogaster; Adipose (brown) Adipose (white) Intestine, large Intestine, small Cerebral cortex Olfactory bulb Adrenal gland Lacrimal gland Mammary gland Salivary gland Mast cells- IgE
dmOTOPLa 5
ce, Caenorhabditis elegans.(B) Distribution dmOTOPLb
of Otop genes in selected murine tissues dmOTOPLc
from microarray data (16). Scale ceOTOPL1
represents expression level in mOTOP2
arbitrary units (mean ± SEM, n = 2). pH 6 5.5 5 4.5 4 7.4 1.0 0
(C, F, I) Representative traces
0.5
(V m = –80 mV) showing currents evoked in -80 mV V (mV)
Xenopus oocytes expressing OTOP2, -80 40 80 I (-µA)
OTOP3, or dmOTOPLc in response to 7.4 Δ 1
+
varying pH o of the Na -free extracellular 4-6 I (A) µ
200 nA -1.0
solution. (D, G, J) I-V relationship
20 s 7 6 5 4
(from voltage ramps at 1 V/s) from pH
experiments as in (C), (F), and (I). mOTOP3
(E, H, K) The average current induced at pH 6 5.5 5 4.5 4 7.4 V (mV) 0
V m = –80 mV (DI) as a function 7.4, 6 -40 40 80
5.5 1
of pH for oocytes expressing -1
5 I (-µA) Downloaded from
each of the channels (black circles; 500 nA
4.5 -2 Δ 2
mean ± SEM, n = 3 to 7) and 30 s I (A) µ
4
for uninjected oocytes (gray triangles, -3 3
mean ± SEM, n = 3). 6 5 4
pH
dmOTOPLc
pH 6 5.5 5 4.5 4 7.4 V (mV) 0
7.4 -40 40 80
6
-0.5 1
5.5 I (-µA) http://science.sciencemag.org/
5 -1.0
500 nA I (A) µ Δ
-1.5 2
30 s 4.5,4
6 5 4
pH
providing evidence that, like OTOP1, they form similar to those of wild-type OTOP1. This reduc- by mice is still speculative and complicated by
proton channels. tion in current magnitude may contribute to the contributions from multiple sensory organs and on March 1, 2018
There are three genes in the genome of Dro- vestibular dysfunction. sensory receptors (19), the identification of OTOP1
sophila melanogaster that encode proteins that Finally, we sought to determine if OTOP1 con- as forming a proton channel provides a tool with
appear to be evolutionarily related to mOTOP1 tributes to the proton current in acid-sensing which to start dissecting this system.
(12, 14) (Fig. 3A). The transcript CG42265 encodes taste receptor cells (10, 11). We confirmed that in Our data show that the otopetrin genes en-
dmOTOPLc, a protein of 1576 amino acids that single-cell transcriptome data, Otop1 was ex- codes a family of ion channels that are unrelated
over the region of similarity bears 14.1% amino pressed in PKD2L1 cells (19 out of 19) implicated structurally to previously identified ion channels
acid identity with OTOP1. Despite the modest level in sour transduction (18), whereas Otop2 and and are highly selective for protons. Unlike Hv1,
of conservation, when expressed in Xenopus oocytes, Otop3 were expressed in much lower amounts, OTOP1 is only weakly sensitive tovoltage. Whether,
dmOTOPLc conducted large currents in response and none of the three Otop transcripts were de- like the viral proton channel M2 (13), low pH
to decreasing extracellular pH, indicating that it tected in TRPM5 cells, which lack proton currents gates OTOP1 is not clear. OTOP channels conduct
too forms a proton channel (Fig. 3, I to K). The (Fig.4A).Byimmunocytochemistry, we con- protons at normal resting potentials and can
shallow relation between the current amplitude firmed that OTOP1 was present in taste cells in mediate the entry of protons into cells. Most cells
and pH may endow the channel with a broader mouse circumvallate papillae that express Pkd2l1 guard against proton entry, which is generally
dynamic range. (Fig. 4B). To directly determine if OTOP1 con- cytotoxic. Thus, we expect that OTOP channels are
OTOP1 is required for the development of tributes to the proton current in taste cells, we restricted to cell types that use changes in intra-
otoconia, calcium carbonate–based structures that measured currents in taste cells from either wild- cellular pH for cell signaling or to regulate bio-
sense gravity and acceleration in the vestibular type mice or mice that were homozygous for chemical or developmental processes. Along
system. Two mutations of Otop1, tilted (tlt) and the tlt mutation of Otop1.Mutation of Otop1 re- with a role in formation of vestibular otoconia
mergulhador (mlh; fig. S11A), lead to vestibular sulted in significantly smaller proton currents (14), OTOP1 has been shown to protect mice from
dysfunction in mice (14). These mutations affect than those measured in taste cells from wild-type obesity-induced metabolic dysfunction (15), and
+
trafficking of the protein to the cell surface in mice (Fig.4,CandD), over arange of H con- it is up-regulated in dorsal root ganglion cells
vestibular supporting cells (17). Mutant channels centrations (Fig. 4, E and F), indicating that in response to cell damage (20). The knowledge
expressed in Xenopus oocytes produced smaller OTOP1 is a component of the proton channel in that this gene family encodes proton channels
currents but otherwise had functional proper- taste cells. Although the contribution of proton can be used to understand its contribution to
ties, such as sensitivity to Zn 2+ (fig. S11, B and C), currents to acid-sensing or sour taste behavior physiology and disease.
Tu et al., Science 359, 1047–1050 (2018) 2 March 2018 3of 4