Page 72 - Maxwell House
P. 72
52 Chapter 2
and such electrically polarized atom acts as a dipole (sensor #2) with dipole moment (see
Figure 2.1.6 and expression (1.1)). Such penomenon is called electronic polarization. Similar
phenomenon is common in more complicated molecule formations when the electron cloud is
shifted to one side of molecule. This causes the whole molecule to become polarized and acts
as s dipole [9].
Another slightly complicated mechanism rules the ionic polarization in materials with ionic
bonding. For the sake of simplicity, consider just two atoms of a different type, for example,
one atom with almost filled up by electrons outermost shell and the second one that has well
only one or two electrons in the same shell. If two such atoms are close enough to each other,
they can join through chemical bonds developing molecules of widely diverse configurations.
It turns out that the atom with one or two electrons quickly gets rid of its outermost electrons
helping another atom to fill up its outermost shell. The atom that gains electrons becomes a
negatively charged ion (anion) because it
now has more electrons than protons.
Alternatively, an atom that loses electrons
becomes a positively charged ion (cation).
Due to this exchange, two atoms usually
form a relatively strong ionic bond,
naturally polarized, and possess nonzero
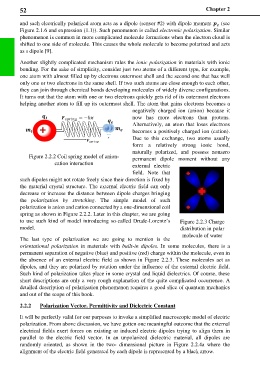
Figure 2.2.2 Coil spring model of anion- permanent dipole moment without any
cation interaction external electric
field. Note that
such dipoles might not rotate freely since their direction is fixed by
the material crystal structure. The external electric field can only
decrease or increase the distance between dipole charges bringing
the polarization by stretching. The simple model of such
polarization is anion and cation connected by a one-dimensional coil
spring as shown in Figure 2.2.2. Later in this chapter, we are going
to use such kind of model introducing so-called Drude-Lorentz’s Figure 2.2.3 Charge
model. distribution in polar
molecule of water
The last type of polarization we are going to mention is the
orientational polarization in materials with built-in dipoles. In some molecules, there is a
permanent separation of negative (blue) and positive (red) charge within the molecule, even in
the absence of an external electric field as shown in Figure 2.2.3. These molecules act as
dipoles, and they are polarized by rotation under the influence of the external electric field.
Such kind of polarization takes place in some crystal and liquid dielectrics. Of course, these
short descriptions are only a very rough explanation of the quite complicated occurrence. A
detailed description of polarization phenomenon requires a good slice of quantum mechanics
and out of the scope of this book.
2.2.2 Polarization Vector. Permittivity and Dielectric Constant
It will be perfectly valid for our purposes to invoke a simplified macroscopic model of electric
polarization. From above discussion, we have gotten one meaningful outcome that the external
electrical fields exert forces on existing or induced electric dipoles trying to align them in
parallel to the electric field vector. In an unpolarized dielectric material, all dipoles are
randomly oriented, as shown in the two- dimensional picture in Figure 2.2.4a where the
alignment of the electric field generated by each dipole is represented by a black arrow.