Page 533 - Most-Essential-Learning-Competencies-Matrix-LATEST-EDITION-FROM-BCD
P. 533
533
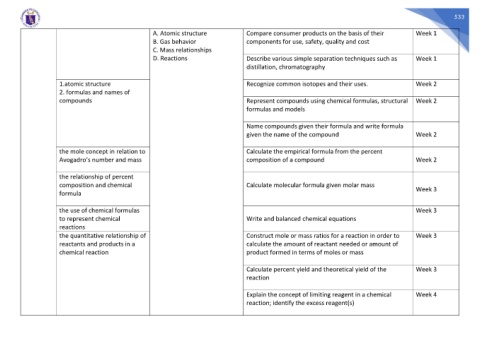
A. Atomic structure Compare consumer products on the basis of their Week 1
B. Gas behavior components for use, safety, quality and cost
C. Mass relationships
D. Reactions Describe various simple separation techniques such as Week 1
distillation, chromatography
1.atomic structure Recognize common isotopes and their uses. Week 2
2. formulas and names of
compounds Represent compounds using chemical formulas, structural Week 2
formulas and models
Name compounds given their formula and write formula
given the name of the compound Week 2
the mole concept in relation to Calculate the empirical formula from the percent
Avogadro’s number and mass composition of a compound Week 2
the relationship of percent
composition and chemical Calculate molecular formula given molar mass Week 3
formula
the use of chemical formulas Week 3
to represent chemical Write and balanced chemical equations
reactions
the quantitative relationship of Construct mole or mass ratios for a reaction in order to Week 3
reactants and products in a calculate the amount of reactant needed or amount of
chemical reaction product formed in terms of moles or mass
Calculate percent yield and theoretical yield of the Week 3
reaction
Explain the concept of limiting reagent in a chemical Week 4
reaction; identify the excess reagent(s)