Page 534 - Most-Essential-Learning-Competencies-Matrix-LATEST-EDITION-FROM-BCD
P. 534
534
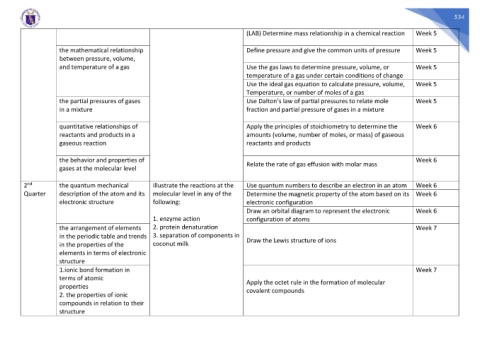
(LAB) Determine mass relationship in a chemical reaction Week 5
the mathematical relationship Define pressure and give the common units of pressure Week 5
between pressure, volume,
and temperature of a gas Use the gas laws to determine pressure, volume, or Week 5
temperature of a gas under certain conditions of change
Use the ideal gas equation to calculate pressure, volume, Week 5
Temperature, or number of moles of a gas
the partial pressures of gases Use Dalton’s law of partial pressures to relate mole Week 5
in a mixture fraction and partial pressure of gases in a mixture
quantitative relationships of Apply the principles of stoichiometry to determine the Week 6
reactants and products in a amounts (volume, number of moles, or mass) of gaseous
gaseous reaction reactants and products
the behavior and properties of Relate the rate of gas effusion with molar mass Week 6
gases at the molecular level
nd
2 the quantum mechanical Illustrate the reactions at the Use quantum numbers to describe an electron in an atom Week 6
Quarter description of the atom and its molecular level in any of the Determine the magnetic property of the atom based on its Week 6
electronic structure following: electronic configuration
Draw an orbital diagram to represent the electronic Week 6
1. enzyme action configuration of atoms
the arrangement of elements 2. protein denaturation Week 7
in the periodic table and trends 3. separation of components in Draw the Lewis structure of ions
in the properties of the coconut milk
elements in terms of electronic
structure
1.ionic bond formation in Week 7
terms of atomic
Apply the octet rule in the formation of molecular
properties
covalent compounds
2. the properties of ionic
compounds in relation to their
structure