Page 536 - Most-Essential-Learning-Competencies-Matrix-LATEST-EDITION-FROM-BCD
P. 536
536
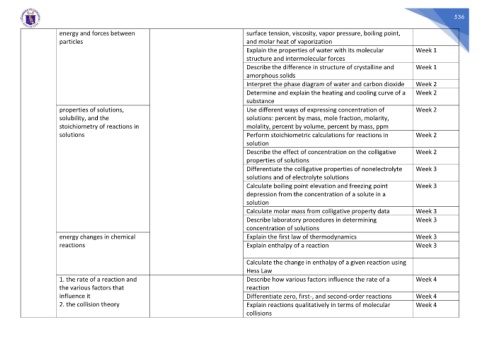
energy and forces between surface tension, viscosity, vapor pressure, boiling point,
particles and molar heat of vaporization
Explain the properties of water with its molecular Week 1
structure and intermolecular forces
Describe the difference in structure of crystalline and Week 1
amorphous solids
Interpret the phase diagram of water and carbon dioxide Week 2
Determine and explain the heating and cooling curve of a Week 2
substance
properties of solutions, Use different ways of expressing concentration of Week 2
solubility, and the solutions: percent by mass, mole fraction, molarity,
stoichiometry of reactions in molality, percent by volume, percent by mass, ppm
solutions Perform stoichiometric calculations for reactions in Week 2
solution
Describe the effect of concentration on the colligative Week 2
properties of solutions
Differentiate the colligative properties of nonelectrolyte Week 3
solutions and of electrolyte solutions
Calculate boiling point elevation and freezing point Week 3
depression from the concentration of a solute in a
solution
Calculate molar mass from colligative property data Week 3
Describe laboratory procedures in determining Week 3
concentration of solutions
energy changes in chemical Explain the first law of thermodynamics Week 3
reactions Explain enthalpy of a reaction Week 3
Calculate the change in enthalpy of a given reaction using
Hess Law
1. the rate of a reaction and Describe how various factors influence the rate of a Week 4
the various factors that reaction
influence it Differentiate zero, first-, and second-order reactions Week 4
2. the collision theory Explain reactions qualitatively in terms of molecular Week 4
collisions