Page 537 - Most-Essential-Learning-Competencies-Matrix-LATEST-EDITION-FROM-BCD
P. 537
537
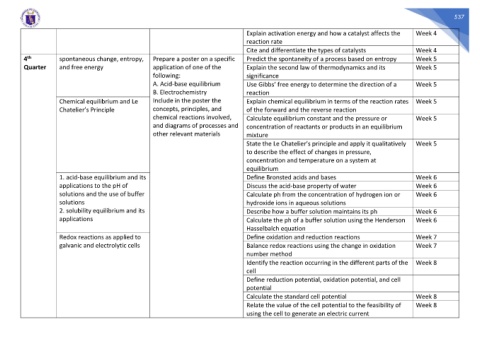
Explain activation energy and how a catalyst affects the Week 4
reaction rate
Cite and differentiate the types of catalysts Week 4
th
4 spontaneous change, entropy, Prepare a poster on a specific Predict the spontaneity of a process based on entropy Week 5
Quarter and free energy application of one of the Explain the second law of thermodynamics and its Week 5
following: significance
A. Acid-base equilibrium Use Gibbs’ free energy to determine the direction of a Week 5
B. Electrochemistry reaction
Chemical equilibrium and Le Include in the poster the Explain chemical equilibrium in terms of the reaction rates Week 5
Chatelier’s Principle concepts, principles, and of the forward and the reverse reaction
chemical reactions involved, Calculate equilibrium constant and the pressure or Week 5
and diagrams of processes and concentration of reactants or products in an equilibrium
other relevant materials mixture
State the Le Chatelier’s principle and apply it qualitatively Week 5
to describe the effect of changes in pressure,
concentration and temperature on a system at
equilibrium
1. acid-base equilibrium and its Define Bronsted acids and bases Week 6
applications to the pH of Discuss the acid-base property of water Week 6
solutions and the use of buffer Calculate ph from the concentration of hydrogen ion or Week 6
solutions hydroxide ions in aqueous solutions
2. solubility equilibrium and its Describe how a buffer solution maintains its ph Week 6
applications Calculate the ph of a buffer solution using the Henderson Week 6
Hasselbalch equation
Redox reactions as applied to Define oxidation and reduction reactions Week 7
galvanic and electrolytic cells Balance redox reactions using the change in oxidation Week 7
number method
Identify the reaction occurring in the different parts of the Week 8
cell
Define reduction potential, oxidation potential, and cell
potential
Calculate the standard cell potential Week 8
Relate the value of the cell potential to the feasibility of Week 8
using the cell to generate an electric current