Page 75 - Pharmaceutics IV (02-06-01305)
P. 75
Parenterals: Containers & packaging
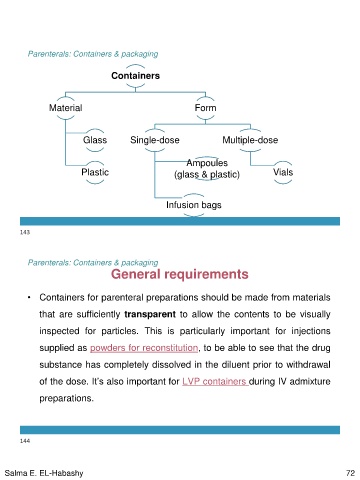
Containers
Material Form
Glass Single-dose Multiple-dose
Plastic
Ampoules Vials
(glass & plastic)
Infusion bags
143
Parenterals: Containers & packaging
General requirements
• Containers for parenteral preparations should be made from materials
that are sufficiently transparent to allow the contents to be visually
inspected for particles. This is particularly important for injections
supplied as powders for reconstitution, to be able to see that the drug
substance has completely dissolved in the diluent prior to withdrawal
of the dose. It’s also important for LVP containers during IV admixture
preparations.
144 72
Salma E. EL-Habashy