Page 34 - Essential Haematology
P. 34
20 / Chapter 2 Erythropoiesis and anaemia
chains in the fetus and adult is discussed in more CH 2
detail in Chapter 7 . The major switch from fetal to CH CH 3
adult haemoglobin occurs 3 – 6 months after birth H
C
(Table 2.2 ; see Fig. 7.1 b). α
H 3 C CH CH 2
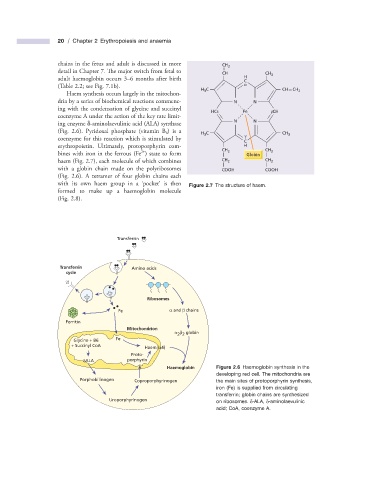
Haem synthesis occurs largely in the mitochon-
dria by a series of biochemical reactions commenc- N N
ing with the condensation of glycine and succinyl
HCδ Fe βCH
coenzyme A under the action of the key rate limit-
N N
ing enzyme δ - aminolaevulinic acid (ALA) synthase
(Fig. 2.6 ). Pyridoxal phosphate (vitamin B 6 ) is a H C CH 3
3
coenzyme for this reaction which is stimulated by γ
C
erythropoietin. Ultimately, protoporphyrin com- H
CH 2 CH 2
2 +
bines with iron in the ferrous (Fe ) state to form Globin
haem (Fig. 2.7 ), each molecule of which combines CH 2 CH 2
with a globin chain made on the polyribosomes COOH COOH
(Fig. 2.6 ). A tetramer of four globin chains each
‘
with its own haem group in a pocket ’ is then Figure 2.7 The structure of haem.
formed to make up a haemoglobin molecule
(Fig. 2.8 ).
Transferrin
Transferrin Amino acids
cycle
Ribosomes
Fe α and β chains
Ferritin
Mitochondrion
α β globin
2 2
Glycine + B6 Fe
+ Succinyl CoA
Haem (x4)
Proto-
δALA porphyrin
Haemoglobin Figure 2.6 Haemoglobin synthesis in the
developing red cell. The mitochondria are
Porphobilinogen Coproporphyrinogen the main sites of protoporphyrin synthesis,
iron (Fe) is supplied from circulating
transferrin; globin chains are synthesized
Uroporphyrinogen on ribosomes. δ - ALA, δ - aminolaevulinic
acid; CoA, coenzyme A.