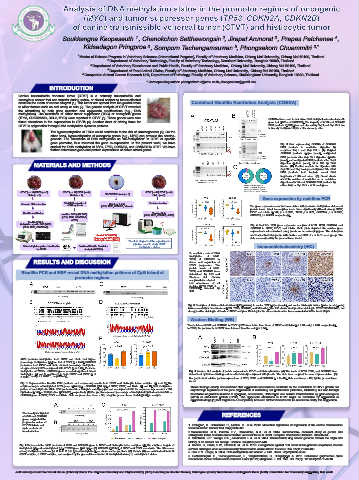
Page 9 - Poster presentation KVAC 22
P. 9
1
2
3
Soukkangna Keopaseuth , Chanokchon Setthawongsin , Jirapat Arunorat , Prapas Patchanee ,
4
Kidsadagon Pringproa , Somporn Techangamsuwan , Phongsakorn Chuammitri 3,*
5
3
1 Master of Science Program in Veterinary Sciences (International Program), Faculty of Veterinary Medicine, Chiang Mai University, Chiang Mai 50100, Thailand
2 Department of Veterinary Technology, Faculty of Veterinary Technology, Kasetsart University, Bangkok 10900, Thailand
3 Department of Veterinary Biosciences and Public Health, Faculty of Veterinary Medicine, Chiang Mai University, Chiang Mai 50100, Thailand
4 Department of Food Animal Clinics, Faculty of Veterinary Medicine, Chiang Mai University, Chiang Mai 50100, Thailand
5 Companion Animal Cancer Research Unit, Department of Pathology, Faculty of Veterinary Science, Chulalongkorn University, Bangkok 10330, Thailand
* Corresponding author: phongsakorn.c@cmu.ac.th, phongsakorn@gmail.com
INTRODUCTION
Canine transmissible venereal tumor (CTVT) is a naturally transmissible and
contagious cancer that can transfer during coitus, or closed contact to tumor-affected
areas as the mean of cancer allograft [1]. This tumor can spread from the genital areas Combined Bisulfite Restriction Analysis (COBRA)
to other tissues such as oral cavity or skin [2]. The genetic analysis of CTVT revealed
the alterations by both gene mutation and epigenetic modifications (e.g., DNA
methylation). The mutations of either tumor suppressor (TSG) or oncogenic genes
(TP53, CDKN2A/2B, BCL2, MYC) were reported in CTVT [3]. These genes were also COBRAs were used to determine DNA methylation levels at specific
found alterations in the expressions in CTVTs [4]. Another facet of driving force for gene loci (MYC and CDKN2B). The majority of MYC and CDKN2B
CTVT is epigenetics through DNA methylation dynamic patterns. PCR products (12/12) were not cleaved by Taq I and Bsp FN I due
to heavily methylated CpGs at the cleavage sites.
The hypermethylation of TSGs could contribute to the risk of carcinogenesis [5]. On the
other hand, hypomethylation of oncogenic genes (e.g., MYC) can provoke the carcino-
genesis. The alterations of DNA methylation as “de-methylation” in CTVT at specific
gene promotor, thus resumed the gene re-expression. In the present work, we have Fig. 3 Gels representing COBRA of CDKN2B
studied the DNA methylation of MYC, TP53, CDKN2A, and CDKN2B in CTVT. We have PCR products to restriction digestion by
further determined gene and protein expressions of above stated genes. enzymes Taq I and Bsp FN I. (A) Original
CDKN2B products (upper panel), undigested
PCR products after Bsp FN I digestion (middle
panel), and undigested PCR products after Taq I
MATERIALS AND METHODS digestion (bottom panel), M = 100 bp DNA
marker. (B) Positive controls for digestion with
Bsp FN I and Taq I using unmethylated 16s rRNA
PCR products from bacteria reveal DNA
fragments of different sizes. (C) Donut charts
show the number of restriction or no restriction
digestion of MYC and CDKN2B PCR products by
either Taq I or Bsp FN I, n = 12 each gene.
GTVT (n = 14) ETVT (n = 6) GTVT (n = 12) ETVT (n = 6) GTVT (n =12) ETVT (n = 6)
Histio (n = 2) Histio (n = 2) CTVT-FFPE block (n = 5) Histio (n = 2)
Gene expression by real-time PCR
Genomic DNA extraction Total RNA extraction & Histology & IHC Protein extraction
cDNA synthesis (MYC, TP53, CDKN2B, Vimentin The gene expressions showed association with promoter methylation status and
protein levels. Most transcription levels were significantly differed among GTVT,
Bisulfite modification Western blots ETVT and Histio (MYC; p < 0.0001, TP53; p = 0.21, CDKN2A; p = 0.0029,
real-time PCR (qRT-PCR) (MYC, TP53, CDKN2B, ACTB) CDKN2B; p < 0.0001, respectively).
(MYC, TP53, CDKN2A/B,
ACTB)
Bisulfite PCR &
Methylation specific PCR (MSP) Fig. 4 Real-time PCR gene expression analysis of MYC, TP53, CDKN2A, and
(MYC, TP53, CDKN2A/B) CDKN2B in GTVT, ETVT, and Histio. Violin plots depicted the relative gene
expression level normalized using β-actin as housekeeping gene. The violin plots
Genital TVT (GTVT), show smoothed histograms with median and IQR of n = 3−11 each group. The
Extragenital TVT (ETVT) data are analyzed by the general linear model.
Histiocytic tumor (Histio) The flow diagram of the experimental
pipeline used to study CTVT
DNA methylation pattern identification Combined Bisulfite Restriction methylation status Immunohistochemistry (IHC)
by QUMA Analysis (COBRA)
To confirm a role of DNA
methylation of MYC,
TP53, & CDKN2B in
RESULTS AND DISCUSSION driven and regulation of
CTVT progression. The
protein levels of MYC,
Bisulfite PCR and MSP reveal DNA methylation patterns of CpG island at TP53, and CDKN2B were
determined by IHC and
promoter regions. Western blot. Protein
analysis demonstrated
low abundance of all
proteins (MYC, TP53, &
CDKN2B) detected by
IHC.
Fig. 5 Examples of immunohistochemical (IHC) staining in canine TVT (upper panels) and canine histiocytic tumor (lower panels) using
immunoreactivity to four markers (MYC, TP53, CDKN2B, and Vimentin). The IHC shows weak positive staining in MYC/TP53/CDKN2B, but
strong positive staining in vimentin of CTVT samples. Staining for the above markers has been evaluated at 400×, bar = 20 μm.
Western Blotting (WB)
The proteins of MYC and CDKN2B in ETVT by WB were lower than those of GTVT and Histio (p = 0.09 and p = 0.04, respectively).
In TP53, the proteins from GTVT were lowered than the rest (p = 0.54).
MYC promoter methylation from GTVT and Histio had higher
percentage methylation (M) than that of ETVT (p = 0.42); CDKN2B
promoters from ETVT and Histio showed percentage methylation
at approximately 80% compared with GTVT (p = 0.49). Promoter
methylation of TP53 or CDKN2A genes revealed significant mixed Fig. 6 Western blot analysis of protein expression in CTVT and histiocytic tumor. (A) The levels of MYC, TP53, and CDKN2B were
methylation (M and U combined) in both GTVT and ETVT (p <
2.2×10 -16 and p < 2.2×10 , respectively). determined by immunoblotting and densitometrically compared with β-actin. The blots were cropped for ease of presentation. (B)
-16
Bar graph show relative protein expressions of MYC, TP53, and CDKN2B (n = 8 –10). Data are means ± SE, by the general linear
model.
Fig. 1 Representative bisulfite PCR products and sequencing results from CTVT and histiocytic tumor samples. (A and C) The
amplicon targets of methylated MYC genes (444 bp) or CDKN2B (354 bp) of GTVT, ETVT, and Histio. (B and D) DNA methylation These findings clearly demonstrated that epigenetic mechanisms contribute to the modulation of CTVT growth not
pattern of each CpG site within PCR products of A or C depicts as lollipop plot and accompanied with the sequence logo consists of 36
CpG sites (MYC) or 15 CpG sites (CDKN2B), respectively. (E) Bar graphs represent percentage of methylated CpG sites from MYC or only through regulation of the gene expressions of controlling cell proliferation (CDKN2A, CDKN2B), but also through
CDKN2B gene of GTVT, ETVT, and Histio. Data are present as mean ± SE, using the general linear model (GLM) for analysis. modulation of proteins involved in apoptosis, senescence, DNA repair, and cellular transformation by proto-oncogene
(MYC) or anti-tumor protein (TP53). The epigenetic alterations in CTVT might be reversible by application of
epigenetic drugs [1] that targets DNA methylation; however, further research will be needed to clarify this suggestion.
The heavily methylated
at MYC and CDKN2B REFERENCES
genes suggested the
progressive nature of
ETVT/ Histio in cell 1. Frampton, D., Schwenzer, H., Marino, G., et al. 2018. Molecular signatures of regression of the canine transmissible
cycle control and venereal tumor. Cancer Cell. 33(4):620-633.
transformation.
2.Mascarenhas, M.B., Peixoto, P.V., Ramadinha, R.R., et al. 2014. Immunohisto- chemical study of genital and
extragenital forms of canine transmissible venereal tumor in Brazil. Pesquisa Veterinária Brasileira. 34:250-254.
3. Murchison, E.P., Wedge, D.C., Alexandrov, L.B., et al. 2014. Transmissible dog cancer genome reveals the origin and
history of an ancient cell lineage. Science. 343(6169):437-440.
Fig. 2 Representative MSP products of TP53 and CDKN2A gene in CTVT and histiocytic tumor samples. (A) The amplicon targets of 4. Decker, B., Davis, B.W., Rimbault, M., et al. 2015. Comparison against 186 canid whole-genome sequences reveals
methylated (M) and unmethylated (U) of TP53 gene (446 bp) or (B) CDKN2A (106 bp) of GTVT and ETVT are shown. The differences survival strategies of an ancient clonally transmissible canine tumor. Genome Res. 25(11):1646-165.
among methylation patterns [M, U, both M & U (mixed methylation)] are shown as bar graphs in (C). Data in (C) are summarized from 14 5. Das, P.M., Singal, R. 2004. DNA methylation and cancer. J. Clin. Oncol. 22(22):4632-4642.
GTVT samples, 6 ETVT samples, and analyzed by the general linear model. M, methylated status; U, unmethylated status. 6. Setthawongsin, C., Techangamsuwan, S., Tangkawattana, S., Rungsipipat, A. 2016. Cell-based polymerase chain
reaction for canine transmissible venereal tumor (CTVT) diagnosis. J. Vet. Med. Sci. 78(7): 10.1292/jvms.15-0710.
Acknowledgement: We would like to gratefully thank The Improved Sanitary and Phytosanitary (SPS) Handling in Greater Mekong Subregion (GMS) Trade/Asian Development Bank (ADB) Foundation for financially supporting this work.