Page 125 - Efavirenz WHO PQ: A case study of a public-private collaboration
P. 125
ภาคผนวก 2 สรุปบทเรียน การด าเนินงานในโครงการ WHO PQ ของ ดร.ภญ.มุกดาวรรณ ประกอบไวทยกิจ | 107
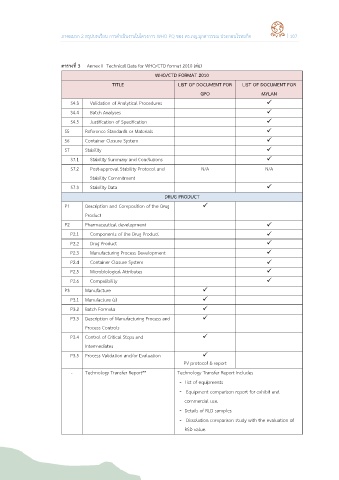
ตารางที่ 3 Annex II Technical Data for WHO/CTD format 2010 (ต่อ)
WHO/CTD FORMAT 2010
TITLE LIST OF DOCUMENT FOR LIST OF DOCUMENT FOR
GPO MYLAN
S4.3 Validation of Analytical Procedures
S4.4 Batch Analyses
S4.5 Justification of Specification
S5 Reference Standards or Materials
S6 Container Closure System
S7 Stability
S7.1 Stability Summary and Conclusions
S7.2 Post-approval Stability Protocol and N/A N/A
Stability Commitment
S7.3 Stability Data
DRUG PRODUCT
P1 Description and Composition of the Drug
Product
P2 Pharmaceutical development
P2.1 Components of the Drug Product
P2.2 Drug Product
P2.3 Manufacturing Process Development
P2.4 Container Closure System
P2.5 Microbiological Attributes
P2.6 Compatibility
P3 Manufacture
P3.1 Manufacture (s)
P3.2 Batch Formula
P3.3 Description of Manufacturing Process and
Process Controls
P3.4 Control of Critical Steps and
Intermediates
P3.5 Process Validation and/or Evaluation
PV protocol & report
- Technology Transfer Report** Technology Transfer Report includes
- List of equipments
- Equipment comparison report for exhibit and
commercial use.
- Details of RLD samples
- Dissolution comparison study with the evaluation of
RSD value.