Page 123 - Feed Lab
P. 123
Suguna Management System Ver 1.0 / SOP / FQL / P2 - 34 Page 1 of 1
34. Estimation of Solubility of
Phosphorus in Citric Acid
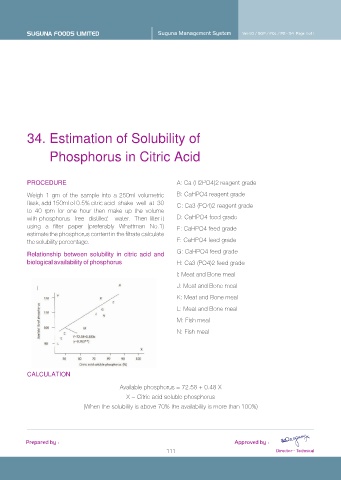
A: Ca (H2PO4)2 reagent grade
PROCEDURE
Weigh 1 gm of the sample into a 250ml volumetric B: CaHPO4 reagent grade
flask, add 150ml of 0.5% citric acid shake well at 30
C: Ca3 (PO4)2 reagent grade
to 40 rpm for one hour then make up the volume
with phosphorus free distilled water. Then filter it D: CaHPO4 feed grade
using a filter paper (preferably Whattman No.1) E: CaHPO4 feed grade
estimate the phosphorus content in the filtrate calculate
the solubility percentage. F: CaHPO4 feed grade
G: CaHPO4 feed grade
Relationship between solubility in citric acid and
H: Ca3 (PO4)2 feed grade
biological availability of phosphorus
I: Meat and Bone meal
J: Meat and Bone meal
K: Meat and Bone meal
L: Meat and Bone meal
M: Fish meal
N: Fish meal
CALCULATION
Available phosphorus = 72.58 + 0.48 X
X = Citric acid soluble phosphorus
(When the solubility is above 70% the availability is more than 100%)
Prepared by : Approved by :
111 Director - Technical