Page 2832 - Cote clinical veterinary advisor dogs and cats 4th
P. 2832
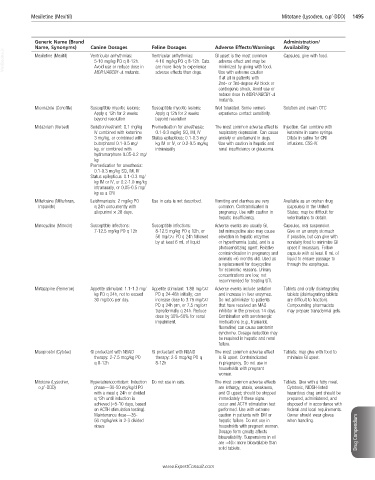
Mexiletine (Mexitil) Mitotane (Lysodren, o,p’-DDD) 1495
Generic Name (Brand Administration/
Name, Synonyms) Canine Dosages Feline Dosages Adverse Effects/Warnings Availability
VetBooks.ir Mexiletine (Mexitil) Ventricular arrhythmias: Ventricular arrhythmias: GI upset is the most common Capsules; give with food.
5-10 mg/kg PO q 8-12h.
adverse effect and may be
4-10 mg/kg PO q 8-12h. Cats
Avoid use or reduce dose in
minimized by giving with food.
are more likely to experience
MDR1/ABCB1-Δ mutants.
adverse effects than dogs.
Use with extreme caution
if at all in patients with
2nd- or 3rd-degree AV block or
cardiogenic shock. Avoid use or
reduce dose in MDR1/ABCB1-Δ
mutants.
Miconazole (Conofite) Susceptible mycotic lesions: Susceptible mycotic lesions: Well tolerated. Some owners Solution and cream OTC
Apply q 12h for 2 weeks Apply q 12h for 2 weeks experience contact sensitivity.
beyond resolution beyond resolution
Midazolam (Versed) Sedation/restraint: 0.1 mg/kg Premedication for anesthesia: The most common adverse effect is Injection. Can combine with
IV combined with ketamine 0.1-0.3 mg/kg SQ, IM, IV respiratory depression. Can cause ketamine in same syringe.
3 mg/kg, or combined with Status epilepticus: 0.1-0.3 mg/ anxiety or excitement in dogs. Dilute in saline for CRI
butorphanol 0.1-0.5 mg/ kg IM or IV, or 0.2-0.5 mg/kg Use with caution in hepatic and infusions. CSS-IV.
kg, or combined with intranasally renal insufficiency or glaucoma.
hydromorphone 0.05-0.2 mg/
kg
Premedication for anesthesia:
0.1-0.3 mg/kg SQ, IM, IV
Status epilepticus: 0.1-0.3 mg/
kg IM or IV, or 0.2-1.0 mg/kg
intranasally, or 0.05-0.5 mg/
kg as a CRI
Miltefosine (Milteforan, Leishmaniasis: 2 mg/kg PO Use in cats is not described. Vomiting and diarrhea are very Available as an orphan drug
Impavido) q 24h concurrently with common. Contraindicated in (capsules) in the United
allopurinol × 28 days. pregnancy. Use with caution in States; may be difficult for
hepatic insufficiency. veterinarians to obtain.
Minocycline (Minocin) Susceptible infections: Susceptible infections: Adverse events are usually GI, Capsules, oral suspension.
7-12.5 mg/kg PO q 12h 8-12.5 mg/kg PO q 12h, or but minocycline also may cause Give on an empty stomach
50 mg/CAT PO q 24h followed elevation in hepatic enzymes if possible, but can give with
by at least 6 mL of liquid or hyperthermia (cats), and is a nondairy food to minimize GI
photosensitizing agent. Relative upset if necessary. Follow
contraindication in pregnancy and capsule with at least 6 mL of
animals <6 months old. Used as liquid to ensure passage to
a replacement for doxycycline through the esophagus.
for economic reasons. Urinary
concentrations are low; not
recommended for treating UTI.
Mirtazapine (Remeron) Appetite stimulant: 1.1-1.3 mg/ Appetite stimulant: 1.88 mg/CAT Adverse events include sedation Tablets and orally disintegrating
kg PO q 24h, not to exceed PO q 24-48h initially; can and increase in liver enzymes. tablets (disintegrating tablets
30 mg/DOG per day. increase dose to 3.75 mg/CAT Do not administer to patients are difficult to fraction).
PO q 24h prn, or 7.5 mg/CAT that have received an MAO Compounding pharmacists
transdermally q 24h. Reduce inhibitor in the previous 14 days. may prepare transdermal gels.
dose by 30%-50% for renal Combination with serotonergic
impairment. medications (e.g., tramadol,
fluoxetine) can cause serotonin
syndrome. Dosage reduction may
be required in hepatic and renal
failure.
Misoprostol (Cytotec) GI protectant with NSAID GI protectant with NSAID The most common adverse effect Tablets; may give with food to
therapy: 2-7.5 mcg/kg PO therapy: 2-5 mcg/kg PO q is GI upset. Contraindicated minimize GI upset.
q 8-12h 8-12h in pregnancy. Do not use in
households with pregnant
women.
Mitotane (Lysodren, Hyperadrenocorticism: Induction Do not use in cats. The most common adverse effects Tablets. Give with a fatty meal.
o,p’-DDD) phase—30-50 mg/kg/d PO are lethargy, ataxia, weakness, Cytotoxic, NIOSH-listed
with a meal q 24h or divided and GI upset; should be stopped hazardous drug and should be
q 12h until induction is immediately if these signs prepared, administered, and
achieved (≈5-10 days, based occur and ACTH stimulation test disposed of in accordance with
on ACTH stimulation testing). performed. Use with extreme federal and local requirements.
Maintenance dose—35- caution in patients with DM or Owner should wear gloves
50 mg/kg/wk in 2-3 divided hepatic failure. Do not use in when handling.
doses households with pregnant women.
Dosage form greatly affects Drug Compendium
bioavailability. Suspensions in oil
are ≈40× more bioavailable than
solid tablets.
www.ExpertConsult.com